Purpose: To determine the total hardness concentration in drinking water from 4 different sources, plus a Saskatchewan Guideline Limit Sample (SGLS) for quality control purposes:
Urban treated water
Rural (Aboriginal and/or non-Aboriginal community) treated water
Untreated raw source water
Local community treated water
Saskatchewan Guideline Limit Sample (SGLS) for total hardness
Determination will be done by using a test strip method. You will compare the different results, you will also see if the water meets the Saskatchewan Guideline Limit Sample (SGLS).
Total hardness is a measurement of calcium, magnesium, and is expressed as calcium carbonate; our body needs both Ca and Mg to remain healthy. The direct health effects are unknown because very little research has been done on the effects of hard water consumption. The major concern with elevated levels of hardness is scale depositing on piping, and drains which makes them less efficient. If water is too hard it will also decrease the washing ability of many soaps and detergents (the soap may not clean properly), as well as affect the taste of the water.
Materials:
1 – An 800 mg/L Total Hardness (SGLS)
5 - Test strip packets (with colour charts printed on them)
5 - 10 mL disposable beakers.
Method:
Label five beakers with their respective names.
Put 10 mL of sample in their respective beakers.
Dip one test strip in sample beaker for 3 seconds.
Remove and immediately match to the closest colour on the colour chart that is located on the test strip packet. Colour is only stable for 1 minute.
Read results as mg/L (parts per million), match with the best colour to determine the total hardness concentration.
Record Your Results
Results: The Saskatchewan Guideline Limit Sample (SGLS) for Total Hardness should give a result very close to the 800 mg/L guideline; this is a very high level of hardness and should only be encountered in untreated well water sources.
Safe Handling of Materials
Caution must be taken at all times when handling any chemicals. Although this test is safe to use in any area, please be cautious with the materials supplied.
Total Hardness:
What is total hardness and why do we test our water for it?
The guidelines for hardness are based on aesthetic, rather than health concerns. Hard water causes scale to form in water pipes, plumbing fixtures and kitchen appliances (see photo). Scale build-up in hot water tanks and boilers increases heating costs and can lead to premature failure of heating equipment. Scale deposited in clothing during washing will cause increased wear and tear on fabrics. Soap reacts with hard water to form a curd and can also cause skin flaking and irritation. In addition, when washing or doing laundry with hard water, more soap or detergent is needed.
Where does hardness in water come from?
Hardness is primarily caused by the dissolved mineral compounds calcium and magnesium although smaller contributions to hardness will also come from some other ions including iron and manganese. The amount of hardness is expressed in milligrams per litre (mg/L) or grains per gallon (gpg) as calcium carbonate.
Hardness is calculated from the equation Hardness = 2.497(Ca) + 4.118(Mg). Therefore, fluctuations in the magnesium pool affect hardness stronger than do calcium fluctuations.
The main components of hardness, calcium and magnesium, are actually of benefit to people. There are no Canadian guidelines for calcium in water and when present in drinking water, calcium may be considered to be of nutritional benefit (if levels around 50 mg/L were consumed, drinking water would provide around 5 to 10% of the daily calcium requirements). The European Community has set a guideline level of 100 mg/L with no maximum acceptable upper concentration. The European Union has also stated that water intended for human consumption should contain a minimum of 20 mg Ca/L.
Magnesium is an essential nutrient for humans, with adults requiring around 350 mg/L per day. Moderate levels of magnesium may provide a nutritional benefit to individuals consuming a magnesium deficient diet. There are no Canadian recommendations in regard to magnesium, but the European Community suggests a guideline of 30 mg/L, with a maximum acceptable level of 50 mg/L, which may be related to magnesium's strong effect on hardness and has no health significance.
What do guidelines say about hardness?
The Guidelines for Canadian Drinking Water Quality notes the following:
Public acceptance of hardness varies considerably. Generally hardness levels between 80 and 100 mg/L as CaCO3 are considered acceptable;
Levels greater than 200 mg/L are considered poor but can be tolerated;
Levels in excess of 500 mg/L are normally considered unacceptable;
Where water is softened by sodium-ion exchange, it is recommended that a separate unsoftened supply be retained for culinary and drinking purposes.
The Saskatchewan Government has set an upper acceptable limit for hardness of 800 mg/L. Such high levels will, however, impart a taste to the water and will cause problems with clothes washing, minerals will be deposited on dishes, tubs and showers and water heaters will become less efficient.
What happens if the hardness is too low or too high?
If the hardness is too low the water can be quite corrosive leaching copper and lead out of plumbing pipes. With very low hardness there would also be low levels of beneficial ions in the water especially calcium and magnesium. If hardness is too high it can have an unpleasant taste, can dry out skin and cause scaling on fixtures and throughout the water distribution system. This scaling is undesirable because it begins to decrease the efficiency of plumbing systems, which results in greater power consumption and increased costs.
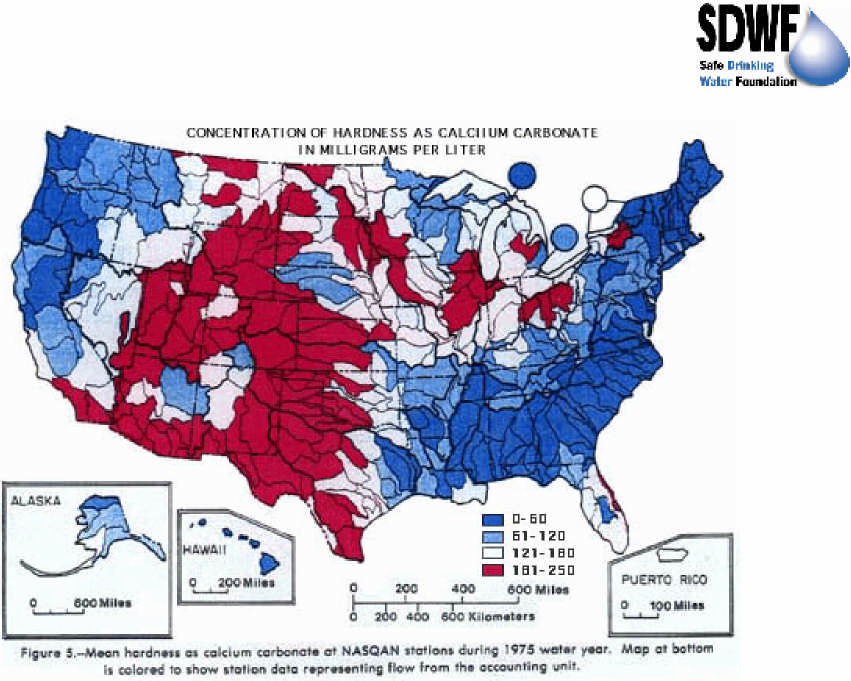
Map of total hardness in water across the United States. It is expected that the shown trends continue into Canada. Source: https://water.usgs.gov/owq/map1.jpeg
What do I do if the level of hardness in my water is too low or too high?
Public water utilities with high hardness levels may not be able to lower these levels as it is difficult to do this and an increased use of membrane technologies will become common in the future. These membranes, such as nanofiltration membranes and reverse osmosis membranes can effectively remove both calcium and magnesium ions from the water (the main causes of hardness). However, when using Reverse Osmosis (which removes virtually all calcium and magnesium ions) it should be borne in mind that the European Union has stated that water intended for human consumption should contain a minimum of 20 mg Ca/L. RO treated water frequently fails to meet this guideline unless calcium is added back to the water. In homes the use of softeners is more common where calcium and magnesium ions are replaced by sodium or potassium, although many homes are now installing under the sink reverse osmosis membranes to provide drinking water.