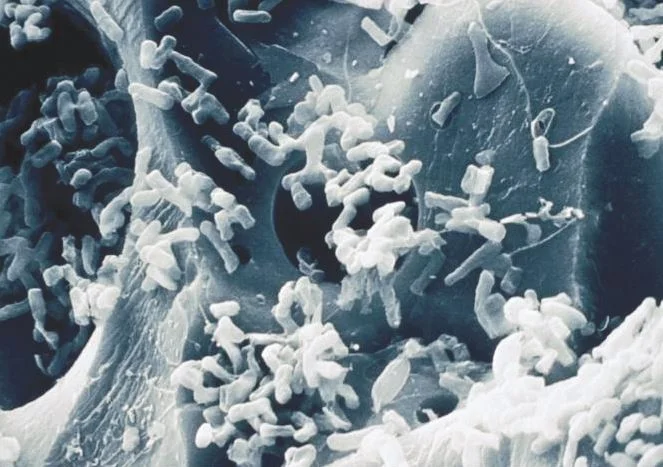
Operation Water Biology
Operation Water Biology (OWB) is a series of eight lesson plans designed for use with students in grades 9-12. OWB directly connects with science, chemistry and biology curricula and covers several different aspects of drinking water treatment. The major topics are chlorine, chloramine, ammonia and iron. For each of these there is a discussion explaining what it is and its importance to drinking water treatment. There are also lab activities for each which allow students to work with small amounts of these substances and see them in action. Students will demonstrate the idea of chlorine demand, create chloramine through a simple chemical reaction, test local samples of drinking water for chlorine and ammonia, and filter water samples with iron oxidized by different processes to determine if one is superior. Every lesson includes additional suggested activities and resources, along with references to other sources of information.
Generally, sponsored Operation Water Biology kits are sent to schools every second Monday from mid-September until the beginning of November and the beginning of March until the end of May. Purchased kits are usually sent on the same dates, but teachers can request that they be sent as soon as possible and we will do our best to accommodate their request.
Cost
The cost of an Operation Water Biology kit is $170 and includes all of the materials necessary to conduct the experiments. Many school kits are available free of charge as a result of different sponsors. However, if there is not a sponsored kit available for your school or you want a kit right away it is best to purchase a kit.
Or, phone us at 1-306-934-0389 to pay for your kit with Visa or MasterCard.
Or, mail a cheque payable to Safe Drinking Water Foundation to:
Safe Drinking Water Foundation
#1-912 Idylwyld Drive North
Saskatoon, SK S7L 0Z6
Overview Materials
Alberta Grade Nine Science Unit C: Environmental Chemistry (Social and Environmental Emphasis) 1: Investigate and describe, in general terms, the role of different substances in the environment in supporting or harming humans and other living things
A list of the materials which are contained in each Operation Water Biology kit. Lesson 1 bag: 6 total chlorine test strips, 6 plastic cups, 6 empty 5mL vials, One tube of Granular Activated Carbon (GAC)
Along with the materials provided in the Operation Water Biology (OWB) kits, and the information and instructions provided in the handouts, there are a number of additional things the teacher will be asked to do or provide in order for the class to fully participate in the lessons.
These are the directions for performing the tests that you will refer to each time you need to determine the total chlorine, free chlorine or ammonia concentration of a water sample.It is important to follow these testing instructions very closely. Extra test strips are not provided so you will need to make each one count.
You are a water treatment plant operator for your city’s local water treatment plant. Your job is to watch the water’s chemical composition for any chemicals that could be dangerous to your city’s inhabitants. This means that you conduct multiple types of tests on the water on a regular basis in order to collect the data you need.
In a First Nations community not far from Edmonton, there has been a boil water advisory in place for many years. The groundwater has been contaminated with iron and arsenic for a long time and they do not have a working water treatment facility in place.
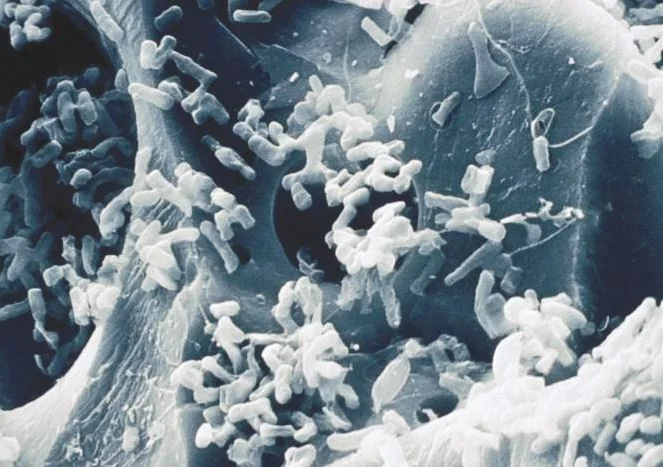
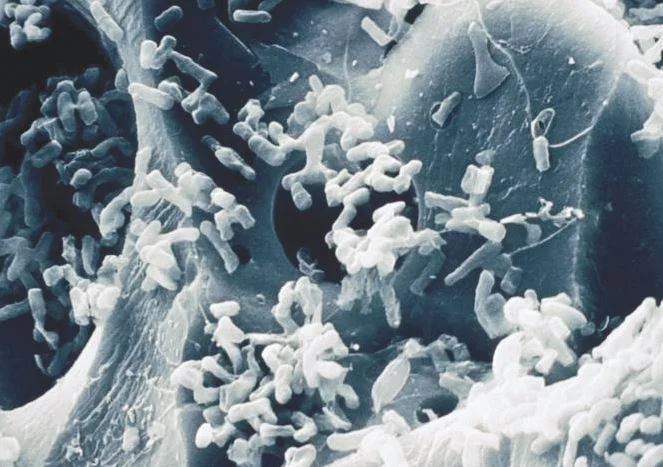
Biological Filtration: The use of bacteria and natural biological processes to remove contaminants from water.
Bio-oxidization: When a substance has been oxidized by bacteria.
Operation Water Biology Lesson One Chlorination and Dechlorination The purpose of a water treatment plant is to take raw water form a well or fresh water source, remove all of the contaminants and make the water safe to drink.
Read an Material Safety Data Sheet (MSDS) for granular activated carbon and learn why ITS test strips do not require MSDS sheets.
Lesson 1: Lesson and Related Resources
The students will gain a basic understanding of the role of water treatment facilities. Students will learn about the importance of eliminating biological activity in drinking water. Students will learn about chlorination as a means of disinfecting water. Students will learn about the concept of dechlorinating water and one of the ways this can be done.
What is the purpose of a water treatment plant? To remove all contaminants and make the water safe to drink
What should be removed? Bacteria, ammonia, phosphorus, nitrogen, dissolved organic material, iron, arsenic, and more….
What is the role of the water treatment facility? Elimination of biological activity in water, Chlorination as a means of disinfecting water, Dechlorination of water – one method
Lesson 2: Lesson and Related Resources
Students will expand their understanding of chlorine demand by participating in an experiment. Students will learn about the Guidelines for Canadian Drinking Water Quality guideline for total chlorine. Students will learn how to perform calculations involving concentration, volume and dilution factors.
Chlorine gets used up when disinfecting water
The total amount of chlorine that must be added to the water to disinfect it is its chlorine demand.
Would good water have a high or a low chlorine demand?
The United States Environmental Protection Agency’s maximum residual disinfectant level goal for chlorine is 4 ppm.
Lesson 3: Lesson and Related Resources
Students will learn about the sources of ammonia in ground water, the consequences of high ammonia concentrations in water supplies, and the manner in which these problems are usually dealt with. Students will learn about biological filtering as a way to use bacteria to remove ammonia from water. Students will learn about the chemical reaction between chlorine and ammonia which results in the creation of chloramine and will learn about chloramine. Students will be able to demonstrate calculations involving concentrations, volumes and dilution factors.
1. Which chemical is one of the most important in the water treatment process?
2. What is the chemical formula for ammonia?
3. Which two types of water sources commonly contain ammonia?
4. Give 3 ways that ammonia can be added to soil.
Lesson 4
Students will learn more about chemical reactions involved in the water treatment process by participating in an experiment examining these reactions. Students will collect and analyze experimental data on chemical reactions. Students will be able to demonstrate calculations involving concentrations, volumes and dilution factors.
Lesson 5: Lesson and Related Resources
Students will expand their knowledge of the problems caused by ammonia in drinking water sources. Students will investigate the quality of their local water and that of a number of samples from the surrounding area. Students will act to help any communities which are found to have improperly disinfected drinking water.
Consequences of Inadequate Drinking Water Treatment
What problems arise from too much Ammonia in drinking water?
How good is the water around here?
Lesson 6: Lesson and Related Resources
The students will learn about the problems associated with iron in drinking water. Students will learn about the chemical states of iron. Students will learn about removing iron from water using biological treatment processes. Students will gain an appreciation for the use of natural processes to perform tasks that would otherwise require chemicals.
Iron in drinking water Guidelines Iron is essential for humans Where does the iron that we need come from?? Usually from the food that we eat – not necessary in water Canadian Drinking Water Quality GL is 0.3 mg/L
Iron in drinking water Guidelines Iron is essential for humans Where does the iron that we need come from?? Usually from the food that we eat – not necessary in water Canadian Drinking Water Quality GL is 0.3 mg/L
1. Before you added the colour changing reagent, did you think the two unfiltered samples had the same amount of iron? 2. Did you change your mind about this after adding the reagent? 3. Why do you think it is important for this experiment that these two samples have the same iron concentration?
Lesson 7: Lesson and Related Resources
Students will practice research skills. Students will gain a more intimate understanding of a topic that interests them. Students will practice presenting information through both written and oral communication.
Topics to choose from: The Chlorination Process What is it? How is the process used in the treatment of drinking water? How does breakpoint chlorination work? Why is it used?
Grading Rubric for Water Biology Research Project. Students will be graded based on Information on topic (definition, description of process, effects on water quality), use of pictures, and presentation of product.
Lesson 8
Students will gain a better understanding of the process used to treat the drinking water for their community. Students will speak to, and ask questions of, a professional in the field of water treatment.