Desalination Fact Sheet
Many times, during presentations, students ask why we cannot simply desalinate the water from the ocean. After all, whenever you look at the globe, you can see that there is a lot of blue! Why are we being asked to conserve water when water covers about 71% of the Earth’s surface?
Only 3% of the Earth’s water is fresh, and the majority of that is unavailable – it is either locked up in glaciers, in polar ice caps, in the atmosphere, or in the soil, or it is highly polluted or lies too far under the Earth’s surface to be extracted at an affordable cost. 97% of the Earth’s water is found in the oceans. If we could use ocean water to drink, then we would not have a water shortage, correct? Why can we not drink ocean water? There are desalination processes, so why can we not simply desalinate the ocean water if we cannot drink it?
Sodium is a mineral and it is one of the chemical elements that are found in salt. Our bodies need a certain amount of sodium to function. However, too much sodium can be harmful. People cannot drink saline water, but saline water can be turned into freshwater in a process called desalination.
What is Saline Water?
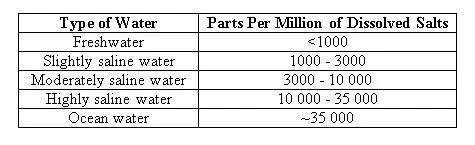
Significant amounts/concentrations of dissolved salts are in saline water. The concentration is the amount (by weight) of salt in water, expressed in ‘parts per million’ or ‘ppm’. If water has a concentration of 10 000 ppm of dissolved salts, then 1% of the weight of the water comes from dissolved salts.
What Happens If You Drink Salt Water?
Drinking salt water can affect your body in a few different ways. Ingesting large quantities of salt causes your cells to lose water by osmosis and, as a result, to shrink. Hypernatremia is a rise in sodium concentration in the blood. Severe hypernatremia can lead to confusion, muscle twitching, seizures, coma, and death. Acute hypernatremia can cause brain damage, and this brain damage can be permanent if the condition is not treated properly. High sodium consumption can increase blood pressure and, in turn, this can harm your kidneys and increase your risk of cardiovascular disease.
Sodium can be beneficial to help reduce inflammation, aid digestion, and promote healthy bones, but the majority of people already get plenty of sodium from the food they eat. Therefore, drinking salt water is unnecessary and could be harmful. The concentration of salt in the ocean is four times higher than the concentration of salt in the human body. Human kidneys can only make urine that is less salty than ocean water. Therefore, to get rid of all the excess salt taken in by drinking ocean water, the person would have to urinate more water than they drank. Eventually, the person would die of dehydration. Too much sodium can cause muscle weakness, restlessness, extreme thirst, and irritability.
What is Desalination?
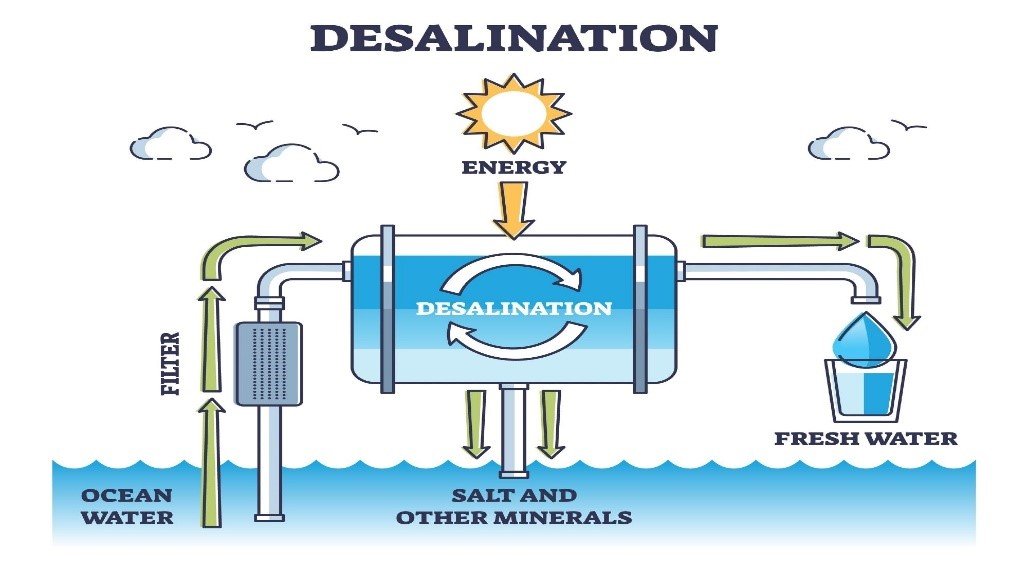
Desalination works by separating the salt molecules from the water molecules. Desalination is any process that removes excess salts and other minerals from ocean water, brackish water, municipal wastewater, and even ground water with high mineral content.
Types of Desalination Processes
There are different desalination processes that are used, such as reverse osmosis and distillation. There are a few different types of distillation such as solar distillation, natural evaporation, vacuum desalination, multi-stage flash distillation, multiple-effect distillation, vapour-compression distillation, wave-powered desalination, and membrane distillation. Of all the possible processes, the most common desalination process is reverse osmosis.
Reverse Osmosis
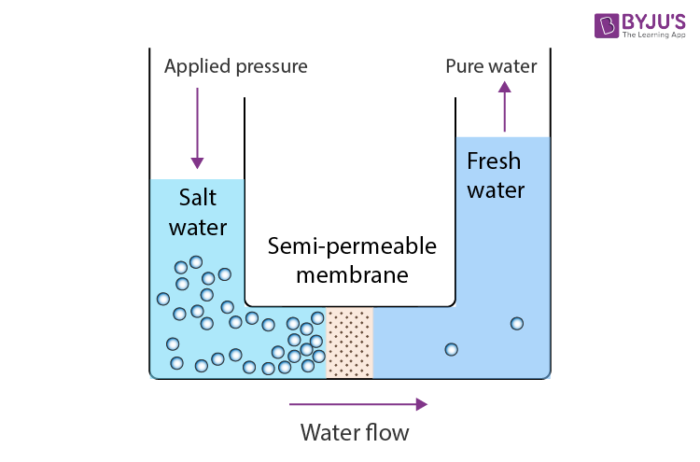
Saline water is pushed through a filter membrane to produce fresh water. This water treatment process uses a pressure gradient to move the high-pressure saltwater feed through a membrane that filters out chlorine, salt, and dirt. Reverse osmosis (RO) desalination uses the principle of osmosis to remove salt and other impurities, by transferring water through a series of semi-permeable membranes.
Osmosis is a simple, natural process that occurs all around us and inside us. In fact, it is one of the most vital processes for our survival! It helps regulate our cells’ lives and helps us to maintain water balance in our bodies. RO is a synthetic process in which a single liquid is forced through a membrane to separate heavier particles from lighter particles. When saline water is forced through a semi-permeable membrane, the heavier salt particles stay on one side and the lighter water molecules pass through the membrane to the other side. You can purchase your own RO system at a local hardware store and some water treatment plants use RO systems and other processes to produce treated drinking water. The downside of RO desalination is that it takes approximately three gallons of saline water to make one gallon of treated drinking water.
Solar Distillation
The power of the sun provides natural distillation. It occurs naturally in the water cycle, when the sun’s heat causes water in oceans and lakes to evaporate before it condenses into clouds. Salts are left behind and natural rain is fresh water.
Vacuum Desalination
Water is vaporized at a lower temperature when subjected to vacuum pressure. The heat energy requirement for desalination using a distillation process can be brought down by reducing the boiling temperature.
Multi-stage Flash Distillation (MSF)
MSF uses heat to convert salt water into fresh water. Sections of MSF systems are called stages, and a normal MSF plant can include anywhere from four to 40 stages, with each consecutive stage functioning at a lower temperature and pressure than the earlier one. This allows the boiling point of the water to decrease as it becomes increasingly concentrated as it leaves the stages. This enables the solution to be brought to a boil multiple times without providing extra heat. During each stage, water vapor forms and is collected. The water vapor is fresh water and the left behind salty concentrate is known as brine. In multistage flash distillation, chemicals or water softening agents are not usually added. MSF is widely used in the Middle East/Gulf area and accounts for 34% of the world’s ocean water desalination.
Multiple-Effect Distillation (MED)
An MED unit is an evaporator in which ocean water is evaporated in one to 14 evaporation stages at low temperature (less than 70° Celsius) to produce clean, distilled water. A vacuum pump is used to reduce the pressure of the first stage to evaporate the hot water. The vapor of the water is condensed on the cooler surface and then collected The main difference between the MSF process and the MED process is the flow direction in the system. In the MSF process, the hot steam and the ocean water flow in opposite directions whereas, in the MED process, they flow in the same direction.
Vapor Compression Distillation
Vapor compression systems use heat that is provided by the compression of vapour rather than by direct heat input from a boiler. When vapour is rapidly compressed, its temperature rises. Some of the compressed and heated vapour is recycled through a series of tubes passing through a reduced-pressure chamber in which evaporation of salt water occurs. This process uses electricity as its main source of energy.
Wave Powered Desalination
Several wave energy converters (WECs) pressurize ocean water, which is then piped ashore to directly drive an ocean water RO desalination system. (Basically, the wave energy converters are series of paddles that are moved forwards and backwards by the waves and this results in energy.)
So, Why Can We Not Desalinate the Ocean Water?
It is possible, desalination does provide around 1% of the world’s drinking water and this percentage is growing every year. However, the cost of the energy to desalinate water would vastly increase the price of water and, as a result, the cost of food. Water would cost approximately 100 times what it does now! Furthermore, the energy it requires would result in a large amount of greenhouse gas emissions.
You might think that the desalination waste would be very useful since people do buy sea salt. However, most desalination processes do not produce sea salt – they produce brine that is very difficult to process. If the brine is discharged into the ocean, it can harm the ecosystem and impede the work of the desalination plant.
Conclusion
It is much easier to conserve water and protect source water than it is to build expensive desalination plants and pay for the energy required to desalinate water. The “solution” of desalinating water would also be much worse for our planet!
Did you know that our Operation Community Water Footprint program includes a math lesson in which students calculate how much raw (source) water is required for their community to produce one litre of treated drinking water and transport it to their tap? Please help us to continue to expand, improve, and update our Operation Community Water Footprint program! Please chip in $5! An Official Donation Receipt for Income Tax Purposes is issued for all donations of $3 or more.
Resources:
Aquatech. (2019, August 12). Wave-powered Desalination – A Cheaper Alternative for Water Security? Aquatech. https://www.aquatechtrade.com/news/desalination/desalination-wave-powered/
Bureau of Reclamation. (2020, November 4). Worldwide Water Supply. Bureau of Reclamation. https://www.usbr.gov/mp/arwec/water-facts-ww-water-sup.html
Encyclopaedia Britannica, Inc. (2021, December 21). Desalination. Encyclopaedia Britannica, Inc. https://www.britannica.com/technology/desalination
Miller, B. (2016, February 3). 12 Biggest Pros and Cons of Desalination. Green Garage. https://greengarageblog.org/12-biggest-pros-and-cons-of-desalination
Muller, R. (2017, September 7). Why Desalination Isn’t The Answer To The World’s Water Problems. Forbes. https://www.forbes.com/sites/lutzfinger/2022/09/08/deepfakesthe-danger-of-artificial-intelligence-that-we-will-learn-to-manage-better/?sh=54d57be6163a
New Health Advisor. (2022, September 29). What Happens When You Drink Salt Water? New Health Advisor. https://www.newhealthadvisor.org/what-happens-when-you-drink-salt-water.html
NOAA. (2021, February 26). Can humans drink seawater? National Ocean Service, National Oceanic and Atmospheric Administration, Department of Commerce. https://oceanservice.noaa.gov/facts/drinksw.html
Rosen, M. & Farsi, A. (2022). Desalination technologies and their working principles. ScienceDirect. https://www.sciencedirect.com/topics/engineering/multi-stage-flash
StudiousGuy. (n.d.). 11 Examples Of Osmosis In Real Life. StudiousGuy. https://studiousguy.com/osmosis-examples-everyday-life/
Water Science School. (2019, September 11). Desalination. U.S. Department of the Interior. https://www.usgs.gov/special-topics/water-science-school/science/desalination#overview
WebMD Editorial Contributors. (2021, April 30). What Is Hypernatremia? WebMD. https://www.webmd.com/a-to-z-guides/what-is-hypernatremia
Winfield, S. (2022, April 13). What is Desalination & How Does It Work? Water Defense. https://waterdefense.org/water-filter/reverse-osmosis/desalination/