ACID RAIN FACT SHEET
What is Acid Rain?
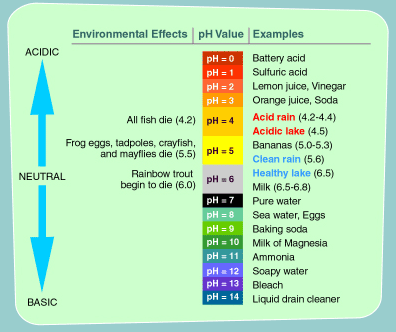
Acid rain is a broad term that is often used to describe several forms of acid deposition. Wet deposition is when rain, snow, fog, or mist contains high amounts of sulfuric and nitric acid. When sulfur dioxide and nitrogen oxides are emitted into the atmosphere, they dissolve in water and fall as precipitation. Dry deposition occurs when dust and smoke that contain high amounts of sulphur dioxide and nitrogen oxides settle to the ground, or onto buildings, cars and vegetation. These gases are converted to acids when they contact water. The acidity of acid rain can vary. Pure water has a pH of 7 and normal rainwater has a pH around 5.6. In 2000, the most acidic rain that fell in the United States had a pH of 4.3.
Where Does Acid Rain Come From?
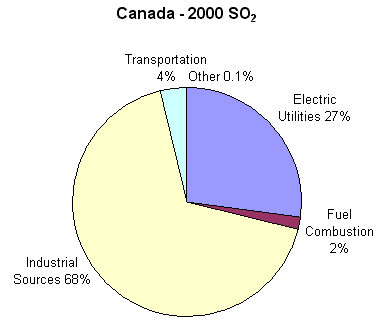
Acid rain develops when sulfur dioxide or nitrogen oxides enter the atmosphere. While natural processes, such as the eruption of a volcano or decomposing vegetation, can emit sulfur dioxide and nitrogen oxides into the air, acid rain is primarily caused by excessive emissions of sulfur dioxide and nitrogen oxides as a result of human actions. Sulfur dioxide is emitted from industrial processes and the burning of fossil fuels. In particular, ore smelting, coal-fired power generators, and the processing of natural gas result in the greatest emissions of sulfur dioxide. In 2000, Canada emitted 2.4 million tonnes of sulfur dioxide; the following graph shows the distribution, by sector.
Sulfur Dioxide Emissions in Canada in 2000 by Sector; https://www.canada.ca/en/environment-climate-change.html
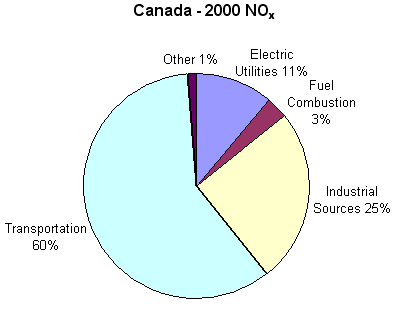
The primary cause of nitrogen oxide emissions are vehicles, which account for about 60 percent of all nitrogen oxide emissions. However, emissions also come from furnaces, boilers and engines. In 2000, Canada emitted 2.5 million tonnes of nitrogen oxide; the following graph shows the distribution, by sector.
Nitrogen Oxide Emissions in Canada in 2000 by Sector; https://www.canada.ca/en/environment-climate-change.html
Does that Mean that Acid Rain Only Occurs in Areas of High Industrial Activity and Transportation?
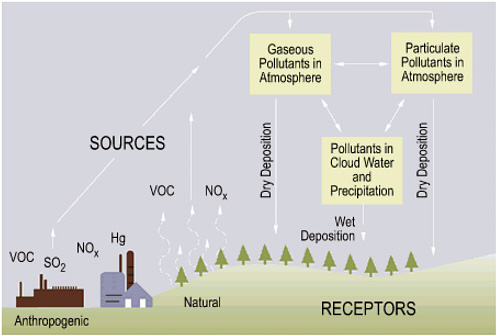
When sulfur dioxide and nitrogen oxides are emitted into the atmosphere, winds can carry them a long way (hundreds of kilometres) before they fall as acid rain. Acid rain is more prevalent in Eastern Canada, but this is due to a combination of factors, including high industrial activity, soil quality and wind directions. The following diagram illustrates the path that sulfur dioxide and nitrogen oxides can take before they fall to the earth as acid rain.
From Source to Acid Rain: How Acid Rain Develops; https://www.nap.edu/openbook/0309089328/xhtml/images/p2000af8eg61001.jpg
What Does Acid Rain Do to the Soil?
Each soil has a buffering capacity, which is the ability of the soil to neutralize acids. An alkaline soil, for example, has a high buffering capacity, because it can absorb high amounts of acidic precipitation without a pH change. Alkaline soil is less harmed by acid rain than sensitive soils with low buffering capacities are. Eastern Canadian soils tend to have lower buffering capacities than western Canadian soils. However, regions of Canada on the Canadian Shield, such as north-eastern Alberta, northern Saskatchewan, northern Manitoba, western British Columbia Nunavut and the Northwest Territories, could be at risk for acid rain, because the granite bedrock cannot effectively neutralize acid. Due to the concentration of industry, the soil quality and the easterly winds, however, eastern Canada receives the majority of acid rain. With the drastic increase in the tar sands mining in northern Alberta, northern Saskatchewan and Alberta could soon be at risk for acid rain. For more information about the tar sands mining, and the extreme environmental degradation that accompanies it, see the https://www.safewater.org/fact-sheets-1/2017/1/23/oil-fields.
What About Lakes and Streams?
Most lakes have pH levels between 6 and 8, but can become acidic, due to acid rain and runoff from soils with low buffering capacities. Alkalinity is a measure of the water’s ability to resist a pH change; it is similar to the buffering capacity of the soil. The amount of acid rain that an environment can absorb without damage is often referred to as the critical load.
Acid rain runoff can also pick up aluminum from the soils and carry it into lakes and streams. Acidic water and high levels of aluminum in the water cause problems for fish and aquatic life, as many are sensitive to small changes. This diagram illustrates the pH levels that various species need to survive. You can see that, at a pH of 6 or 6.5, a wide number of species can survive, but in highly acidic water, the biodiversity is extremely reduced.
Required pH Level for the Survival of Common Species of Fish
The Canadian government estimates that around 14,000 lakes in eastern Canada are acidic. Water sources can also experience episodic acidification, which is when a heavy downpour or runoff from spring melting causes lakes and streams to become temporarily acidic.
Over the past several decades, Norway has suffered great damage due to the effects of acid rain. While Norway’s sulfur dioxide emissions have decreased significantly since the 1970s and 1980s, and nitrogen oxide emissions have decreased slightly, the damages from acid rain appear to be worsening in southern Norway. This is because it takes years for the ecosystems and the environment to recover from the effects of acidification. The following map shows the state of Norway’s fish stocks over 40 years; the red areas are regions in which fish stocks have been completely lost or damaged as a result of acidification. According to the State of the Environment in Norway, 18 salmon stocks have been lost and 12 are endangered, and salmon have been wiped out of all of the large salmon rivers in southern Norway.
Adding lime to water sources can reduce the acidifcation in lakes and rivers, by increasing the buffering capacity and critical load of an environment. Liming is a temporary solution, and is often used only on the most severely damaged lakes and rivers, so that ecosystems have an opportunity to survive and re-build. According to Norway’s State of the Environment, 90 regions in Norway that used liming were studied, and species diversity was deemed to be satisfactory in 85 to 90 percent of the regions. However, liming is an expensive approach to dealing with acidification. The annual cost to lime rivers and lakes in Norway is more than $18 million (in Canadian dollars).
Can Acid Rain Make Drinking Water Unsafe?
Water that is slightly acidic should not be dangerous, as there are many food that have low pH value; for example, lemon juice has a pH of 2.4. However, a low pH can indicate that there may be other contaminants in the water, because if pollutants have been added to a water source, the pH typically will change.
Water treatment facilities monitor the pH level of the water while they are treating it for municipal use. Acidic or basic water is harder to disinfect than water with a pH that is closer t 7.0. As well, if acidic water was sent through pipes and into homes, there would be a greater danger of pipe corrosion, which could allow metals to dissolve into the drinking water as it flow through the pipes. According to the World Health Organization, a pH less than 8.0 is necessary for effective chlorination. If the pH is too high, water treatment facilities can decrease the alkalinity in a number of ways. A variety of aqueous acids can be used to reduce the pH of water. This includes sulphuric acid, hydrochloric acid, nitric acid, and citric acid. Diluted sulphuric or hydrochloric acid are most recommended as they are the most cost effective and can reduce the pH most efficiently.
What Does Acid Rain Do To Vegetation?
Acid rain can weaken trees by damaging the leaves and limiting the amount of available nutrients. Acid rain dissolves nutrients and minerals and carries them away before the vegetation can use them to grow. Crops are not usually harmed by acid rain, because farmers use fertilizer, which includes the necessary nutrients, or add crushed limestone to their fields. Limestone is an alkaline material, so it increases the buffering capacity of the soil to neutralize acids. The picture below shows the effects that acid rain had on a pine tree. The branch on the left has lost needles and turned yellow, which is the result of acid rain.
Comparison Between Tree That Has Been Damaged by Acid Rain and Healthy Tree
Does Acid Rain Harm Buildings?
Acid rain can corrode metals and deteriorate paint and stone. To see the effects of acid rain for yourself, try this experiment: Put a piece of chalk into a bowl white sugar and another into a bowl of tap water. Leave them overnight and see which is more worn away in the morning. Vinegar is an acid with a pH of 2.8, and chalk is made of calcium carbonate, which is a compound of marble and limestone.
Dry deposition can also cause visibility issues, and sulfate particles account for 50 to 70 percent of the visibility reduction in the eastern United States.
Can Acid Rain Cause Health Problems?
There are no direct health issues associated with acid rain. Dry deposition, however, can contribute to heart and lung problems, such as asthma and bronchitis. If eastern Canada and the United States (whose emissions are carried, by the wind, into Canada) were to reduce their sulfur dioxide emissions by 50 percent, it is estimated that Canada could avoid 550 premature deaths, 1,520 emergency room visits, and 210,070 asthma symptom days each year. These savings, depending on how much value society places on these benefits, are worth between $500 million and $5 billion each year!
Well, I Have No Control Over Industry Emissions; What Can I Do?
While industries can use cleaner coal or find alternative fuels to generate power, the best things that individuals can do is conserve energy. Here are a few tips:
Turn off lights, computers and appliances, when you aren't using them.
Use energy efficient appliances.
Turn the thermostat down at night and when you’re away from home.
Insulate your home to reduce air leaks.
Carpool, take the bus, walk or bike to work and school.
Maintain your vehicle.
For more information about acid rain and its effects on the environment, see the lesson plan titled “What is Acid Rain and how does it affect me?" in the Operation Water Flow section.
The Safe Drinking Water Foundation has educational programs that can supplement the information found in this fact sheet. Operation Water Drop looks at the chemical contaminants that are found in water; it is designed for a science class. Operation Water Flow looks at how water is used, where it comes from, and how much it costs; it has lessons that are designed for Social Studies, Math, Biology, Chemistry and Science classes. Operation Water Spirit presents a First Nations perspective of water and the surrounding issues; it is designed for Native Studies or Social Studies classes. Operation Water Health looks at common health issues surrounding drinking water in Canada and around the world and is designed for a Health, Science and Social Studies collaboration. Operation Water Pollution focuses on how water pollution occurs and how it is cleaned up and has been designed for a Science and Social Studies collaboration. To access more information on these and other educational activities, as well as additional fact sheets, visit the Safe Drinking Water Foundation's website at www.safewater.org.
Did you find this information of interest? Please help us to educate more current and future leaders! Please chip in $5 or donate $20 or more and receive an Official Donation Receipt for Income Tax Purposes.
Resources:
Government of Canada.
https://www.canada.ca/en/environment-climate-change.html
State of the Environment Norway. http://www.environment.no/
United States Environmental Protection Agency. March 2017. Acid Rain. https://www.epa.gov/acidrain/what-acid-rain
World Health Organization. 2007. pH in Drinking-water: Revised background document for development of WHO Guidelines for Drinking-water Quality. https://cdn.who.int/media/docs/default-source/wash-documents/wash-chemicals/ph.pdf?sfvrsn=16b10656_4